Mathematics Professor Mary Lou Zeeman’s current research centers on several aspects of the ovulation cycle that continue to puzzle biologists.
Scientists have identified the hormonal changes that cause ovulation, but don’t fully understand how these changes occur on a cycling basis.
“It’s a beautiful cycle between the brain and ovaries,” notes Zeeman. “There are other endocrine feedback networks in the body — the adrenal system for instance operates similarly from the hypothalamus to pituitary to adrenal glands and back — but there are not many that operate on a regular monthly cycle. It generates really thrilling questions: What causes that kind of robust cycling behavior? What keeps it cycling? What is it about the feedback that makes it work or not?”
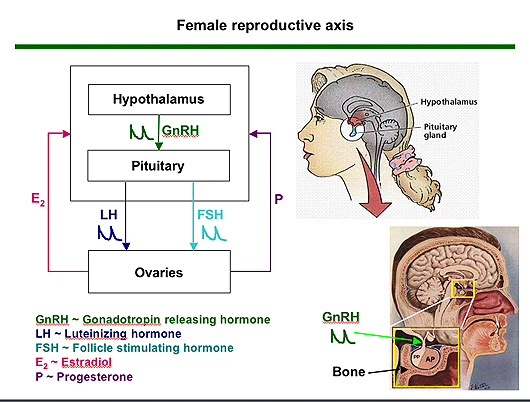
The ovulation cycle is controlled in part by the hypothalamus, which releases pulses of the peptide hormone GnRH, triggering the pituitary gland to release pulses of the hormones (LH and FSH). Those in turn stimulate the egg-containing follicles in the ovary to grow and release the sex hormones estradiol, progesterone and inhibin, which feed back to the brain to significantly change the size and frequency of the GnRH pulses over the course of a cycle. When the egg is mature the estradiol level peaks, and the pituitary releases a dramatic surge of LH, triggering ovulation.
One of the most tantalizing areas of Zeeman’s research concerns the role of the pituitary gland in this feedback cycle. Once referred to as the “master gland” because of its primacy in controlling endocrine systems, it became relegated to the role of “slave” after researchers understood that it actually is responding to pulses of information from the hypothalamus.
“It lost its glory,” jokes Zeeman. “It’s quite funny, so much emotion attached to this little gland. I think that if you read both the medical literature and the scientific literature, it becomes clear that the pituitary is neither master nor slave, but something in between.”
In treatments for women with Kallmann’s Syndrome — a condition in which the woman’s hypothalamus does not produce GnRH — doctors administer regular pulses of GnRH, every 90 minutes, through an arm band. Even though the GnRH pulses do not change in frequency or size, the pituitary gland still produces the surge in LH that triggers ovulation.
This contradicts earlier scientific understanding of the necessity for surging or changing GnRH pulses to trigger the pituitary’s LH surge — putting into question its slave relationship to the hypothalmus.
Further, studies of estrogen-level changes suggest that the gradual, monthly rise of estradiol at first has a dampening effect on LH, then suddenly switches to actually activating the LH surge in the pituitary gland.
“That is the real question that caught my imagination,” says Zeeman, who has been working with Cornell biologist David McCobb to graph these hormonal changes. “Why is it that the estrogen inhibits the LH at the beginning of the menstrual cycle then stimulates it enough to surge? What is the cause of the switch? And what is estrogen doing that allows both steady, regular pulses of administered GnRH and the naturally occurring changing pulses to trigger the LH surge? Those unanswered questions are clearly the reasons why there were no mathematical models for the menstrual cycle.”
All of this has caused the researchers to rethink the pituitary gland altogether.
“The way I like to think about it now is that the pituitary has attitude,” says Zeeman, grinning. “It is a slave with attitude. And its attitude depends on the steroid it is on — because estrogen is a steroid. Putting that a little more formally, the pituitary integrates signals from the hypothalamus and from the ovaries (the target tissue) in an intelligent way so that the pituitary is doing really interesting stuff.”
That “stuff” includes a new theory of the pituitary’s biological functioning. Zeeman and her colleagues are among new researchers who believe the pituitary is neither slave nor master, but acts as an oscillator with its own preferred frequency or rhythm of oscillation.
They posit that the pituitary is integrating hormonal inputs from both the hypothalamus and the ovaries, which cause it to prefer different frequencies over the course of the cycle. When the estrogen released from the ovaries reaches a certain level, the preferred pituitary frequency agrees with the driving frequency of the GnRH pulses, leading to a resonance reaction. This resonance causes the spike in luteinizing (LH) hormone from the pituitary gland, which in turn triggers ovulation.
Their theory is so new that Zeeman and McCobb must first establish its biological basis before gathering the data to analyze it mathematically.
“It’s a whole new and different strand of the research to come up with experiments that can distinguish between different possible mechanisms,” notes Zeeman. “With the mathematical research you can say, okay, here is mechanism A and mechanism B. As soon as you get the equations you can put them in the computer, see how the systems will evolve, and find circumstances in which the different mechanisms make different predictions. But when you are looking for the actual source of the frequency preference it is a very different challenge. How can we reproduce those circumstances biologically to discover which mechanism is actually at play?”
The researchers are experimenting with pituitary tissue from mice, which they run through a perifusion system into which they can pump GnRH, estradiol and other pharmacological agents to test the mechanistic responses of the pituitary.
“As a mathematician, to be able to go in there and collect the data myself is incredible,” says Zeeman. “I could spend the rest of my life analyzing this first set of equations that we have come up with to represent the possible frequency-interaction approach, but I care too much about the real medical question to spend my time analyzing the equations until I am convinced they truly represent the biology.”